Laboratory of Ectopic Olfactory and Taste Receptors (LEO)
Investigation of the novel functions of ectopic olfactory receptors in energy metabolism
Olfactory receptors (ORs) are a class of the G-protein coupled receptors (GPCR) that are primarily expressed in the cilia of the olfactory epithelial cells. It binds with an odorant, and triggers a signal transduction pathways and delivers the odor information to the brain. Recent investigation demonstrated that ORs are also expressed in many extra-nasal tissues and the function of these 'ectopic' ORs are beginning to be emerged. My lab has investigated physiological functions of volatile aroma compounds more than a decade and have hypothesized that aroma odorants may exert biological functions via activation of ectopic ORs in extra-nasal tissues. We have investigated novel functions of ectopic olfactory receptors in energy metabolism during last 6 years.
Olfr43/OR1A1 in hepatocytes
Human OR1A1 is highly expressed in hepatocytes and we demonstrated that (-)-carvone, a major aroma compound in spearmint and a ligand of OR1A1, reduced hepatic lipid accumulation via activation of PKA-CREB-HES1 signaling axis. This suppressed the expression of PPAR-gamma, a lipogenic transcription factor, thus reduced hepatic steatosis. In vivo, Olfr43, a mouse homolog of OR1A1, is highly expressed in mouse livers, and activation of Olfr43 by (-)-carvone significantly improved multiple metabolic parameters related to the energy metabolism and aging: reduction of hepatic steatosis and adiposity, and improved glycemic control. These collectively demonstrated that Olfr43/OR1A1 is a functional receptor in the liver and regulates energy metabolism in vitro and in vivo.
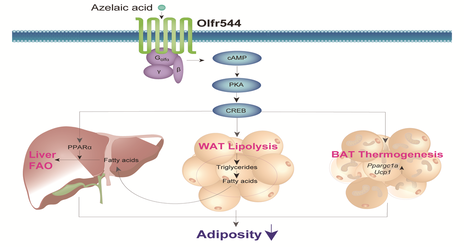
Olfr544 in adiposity
In transcriptome profiling analysis, we identified that mouse olfactory receptor, Olfr544, is widely expressed in various extra-nasal tissues. Interestingly, Olfr544 is the highest OR expressed in the liver, muscle, and adipose tissue. Azelaic acid (AzA) is known as an Olfr544 ligand and stimulation of cultured cells with AzA specifically induced PKA-dependent lipolysis in adipocytes and promoted fatty acid oxidation (FAO) and ketogenesis in liver, thus shifting the fuel preference to fats. Oral administration of AzA induced expression of PPAR-α and genes required for FAO in the liver and induced the expression of PPAR-γ coactivator 1-α (Ppargc1a) and uncoupling protein-1 (Ucp1) genes in brown adipose tissue (BAT). AzA injection induced lipolysis in vivo as well.
Long-term administration of AzA reduced adiposity in both high-fat diet mice and ob/ob mice with increased insulin sensitivity and ketone body levels. Indirect calorimetry analysis showed that AzA lowered RQ ratio, demonstrating the shifting the fuel preference to fats. These demonstrated the novel function of ectopic OR orchestrating the metabolic interplay between the liver and adipose tissue, mobilizing stored fats from adipose tissue and shifting the fuel preference to fats in the liver and BAT.
Olfr43/OR1A1 in hepatocytes
Human OR1A1 is highly expressed in hepatocytes and we demonstrated that (-)-carvone, a major aroma compound in spearmint and a ligand of OR1A1, reduced hepatic lipid accumulation via activation of PKA-CREB-HES1 signaling axis. This suppressed the expression of PPAR-gamma, a lipogenic transcription factor, thus reduced hepatic steatosis. In vivo, Olfr43, a mouse homolog of OR1A1, is highly expressed in mouse livers, and activation of Olfr43 by (-)-carvone significantly improved multiple metabolic parameters related to the energy metabolism and aging: reduction of hepatic steatosis and adiposity, and improved glycemic control. These collectively demonstrated that Olfr43/OR1A1 is a functional receptor in the liver and regulates energy metabolism in vitro and in vivo.
Olfr544 in adiposity
In transcriptome profiling analysis, we identified that mouse olfactory receptor, Olfr544, is widely expressed in various extra-nasal tissues. Interestingly, Olfr544 is the highest OR expressed in the liver, muscle, and adipose tissue. Azelaic acid (AzA) is known as an Olfr544 ligand and stimulation of cultured cells with AzA specifically induced PKA-dependent lipolysis in adipocytes and promoted fatty acid oxidation (FAO) and ketogenesis in liver, thus shifting the fuel preference to fats. Oral administration of AzA induced expression of PPAR-α and genes required for FAO in the liver and induced the expression of PPAR-γ coactivator 1-α (Ppargc1a) and uncoupling protein-1 (Ucp1) genes in brown adipose tissue (BAT). AzA injection induced lipolysis in vivo as well.
Long-term administration of AzA reduced adiposity in both high-fat diet mice and ob/ob mice with increased insulin sensitivity and ketone body levels. Indirect calorimetry analysis showed that AzA lowered RQ ratio, demonstrating the shifting the fuel preference to fats. These demonstrated the novel function of ectopic OR orchestrating the metabolic interplay between the liver and adipose tissue, mobilizing stored fats from adipose tissue and shifting the fuel preference to fats in the liver and BAT.
On-going project
My lab has been elected and funded as a Basic Research Laboratory from National Research Foundation, Korea. In this project, my lab will study the function of ectopic olfactory and taste receptors in brown/beige adipogenesis to further understand complicated biological mechanism in obesity and energy metabolism. Alternatively, ectopic OR has been intensively studied is cancer research since the expression of ORs are highly upregulated in many cancer cells and activation of the ORs could regulate cancer cell activity. Accordingly, we are initiating a novel function of ORs in colon cancer as well.
My lab has been elected and funded as a Basic Research Laboratory from National Research Foundation, Korea. In this project, my lab will study the function of ectopic olfactory and taste receptors in brown/beige adipogenesis to further understand complicated biological mechanism in obesity and energy metabolism. Alternatively, ectopic OR has been intensively studied is cancer research since the expression of ORs are highly upregulated in many cancer cells and activation of the ORs could regulate cancer cell activity. Accordingly, we are initiating a novel function of ORs in colon cancer as well.
Position Opening
We welcome to recruit talented students and post-doc fellows from both Korea and abroad. TOEFL score > 210 (CBT) is recommanded. Please contact the professor, if you want to have more information ( junelee@korea.ac.kr Sung-Joon Lee).
We welcome to recruit talented students and post-doc fellows from both Korea and abroad. TOEFL score > 210 (CBT) is recommanded. Please contact the professor, if you want to have more information ( junelee@korea.ac.kr Sung-Joon Lee).